Concept of Cholera Toxin
The existence of a toxin responsible for cholera symptoms was proposed in 1884 by Robert Koch, who suspected that the agent responsible for cholera produced “a special poison” that acted on the intestinal epithelium and that the cholera symptoms could be “considered as poisoning”. The existence of this hypothetical toxin was demonstrated in 1959 by two independent groups of researchers who worked in India. De et al. and Dutta et al. have shown that a loss of fluid occurred when filtered or lysed Vibrio cholerae cultures were introduced in the intestinal tract of a rabbit. The toxin purification, 10 years later, by Finkelstein and LoSpalluto allowed numerous researchers to discover fundamental properties of this toxin such as its structure, its receptor and its mode of action.
Structure of the cholera Toxin
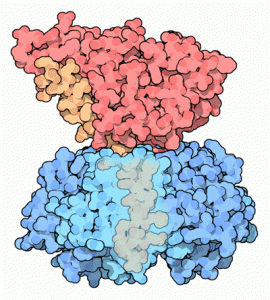
Three-dimensional representation of cholera toxin (Goodsell, D., 2005)
The cholera toxin (CT) is a protein that belongs to the family of type AB toxins. The B subunit serves as the holotoxin carrier until the eukaryotic cell receptor and the subunit A has a specific enzymatic function that acts intracellularly. The cholera toxin has a total molecular weight of about 85.2 KDa. The holotoxin has a molecular weight of about 27.2 KDa. The subunit B, in turn, consists of five identical polypeptide chains of 11.6 KDa, each with 103 amino acids.
The cholera toxin is encoded by a transcription unit, containing the CtxA gene that encodes the subunit A and the CtxB gene that encodes the subunit B, present in the genome of the CTXΦ (CTXphi) phage. The phage genes are transmitted to vibrios by plasmid horizontal transfer and are integrated in the genome of the bacterium, giving it its pathogenicity.
Mechanism of action of cholera Toxin
The intracellular target of cholera toxin is the adenyl cyclase, one of the most important systems of regulation of eukaryotic cells. This enzyme mediates the conversion of ATP to cyclic AMP (cAMP), a crucial intracellular messenger in a wide variety of cellular processes. Normally, the adenyl cyclase is activated or inactivated in response to a variety of stimuli. The regulation of adenylate cyclase is mediated by the G protein, a protein that acts as a link between many surface cellular receptors and the effector protein in the plasma membrane. G proteins are heterotrimers that consist of three distinct subunits: a, b and γ. The specific G protein involved in this case is the Gs protein, which activation leads to an increase in adenyl cyclase activity. The cholera toxin catalyzes the transfer of ADP-ribose from NAD (Nicotinamide Adenine Dinucleotide) to a specific arginine residue in Gs proteina, resulting in the activation of adenyl cyclase and consequently in the increase of intracellular cAMP level. cAMP activates a protein kinase, cAMP-dependent, which leads to protein phosphorylation, to ion transport alteration and finally to diarrhoea.
References:
- Faruque, S.M. and G.B. Nair. 2002. Molecular ecology of toxigenic Vibrio cholerae: Microbiol. Immunol., 46(2):59-66
- Lau, O.L., 2004. Vibrio cholerae: Epidemiology, ecology, evolution and climate change. Reed College, Portland, OR.
- Peterson, K.M. 2002. Expression of V. cholerae virulence genes in response to Environmental signals. Curr. Issues. Microbiol. 3:29 -38




